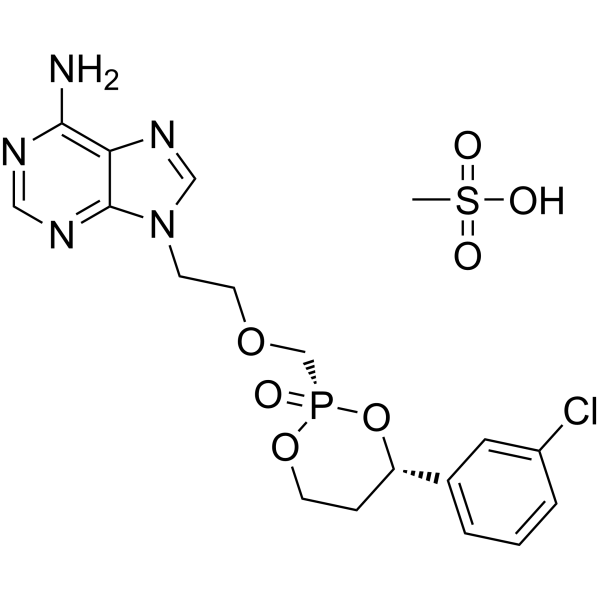
Pradefovir mesylate
CAS No. 625095-61-6
Pradefovir mesylate( Remofovir mesylate | Hepavir B | Pradefovir mesilate )
Catalog No. M27105 CAS No. 625095-61-6
Pradefovir mesylate is converted to 9-(2-phosphonylmethoxyethyl)adenine (PMEA) in human liver microsomes with Km of 60 μM.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 410 | Get Quote |


|
| 10MG | 605 | Get Quote |


|
| 25MG | 954 | Get Quote |


|
| 50MG | 1287 | Get Quote |


|
| 100MG | 1728 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NamePradefovir mesylate
-
NoteResearch use only, not for human use.
-
Brief DescriptionPradefovir mesylate is converted to 9-(2-phosphonylmethoxyethyl)adenine (PMEA) in human liver microsomes with Km of 60 μM.
-
DescriptionPradefovir mesylate is converted to 9-(2-phosphonylmethoxyethyl)adenine (PMEA) in human liver microsomes with Km of 60 μM.(In Vitro):Pradefovir was converted to PMEA in human liver microsomes with a Km of 60 microM, a maximum rate of metabolism of 228 pmol/min/mg protein, and an intrinsic clearance of about 359 ml/min.?Addition of ketoconazole and monoclonal antibody 3A4 significantly inhibits the conversion of pradefovir to PMEA in human liver microsomes, suggesting the predominant role of CYP3A4 in the metabolic activation of pradefovir.?Pradefovir at 0.2, 2, and 20 microM was neither a direct inhibitor nor a mechanism-based inhibitor of CYP3A4, CYP2D6, CYP2C9, CYP2C19, CYP2E1, and CYP1A2 in human liver microsomes.(In Vivo):In rats, the liver was the site of metabolic activation of pradefovir, whereas the small intestine did not play a significant role in the metabolic conversion of pradefovir to PMEA.?Daily oral dosing (300 mg/kg) to rats for 8 days showed that pradefovir was not an inducer of P450 enzymes in rats.?
-
In VitroPradefovir is a cyclodiester prodrug of PMEA. It is one of the Hep Direct prodrugs, which are designed to be efficiently and specifically activated through an oxidative reaction catalyzed by CYP3A4, which is located mainly in the liver.Pradefovir is converted to PMEA in human liver microsomes with a Km of 60 μM, a maximum rate of metabolism of 228 pmol/min/mg protein, and an intrinsic clearance of about 359 L/min.
-
In VivoDaily oral dosing of Pradefovir (300 mg/kg) to rats for 8 days does not affect body weight; liver weight; liver weight-body weight ratio; liver microsomal protein content; total CYP content; enzyme activities for CYP1A, CYP2B, and CYP3A; and apoprotein contents for CYP1A1, CYP2B1/2, CYP3A1/2, and CYP4A1/3, indicating that Pradefovir is not a CYP inducer in rats.
-
SynonymsRemofovir mesylate | Hepavir B | Pradefovir mesilate
-
PathwayMetabolic Enzyme/Protease
-
TargetP450
-
RecptorACAT
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number625095-61-6
-
Formula Weight519.89
-
Molecular FormulaC18H23ClN5O7PS
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?H2O : 100 mg/mL (192.34 mM)
-
SMILESCS(O)(=O)=O.Nc1ncnc2n(CCOC[P@@]3(=O)OCC[C@H](O3)c3cccc(Cl)c3)cnc12
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference



-
N-Nornuciferine hydr...
N-Nornuciferine is an aporphine alkaloid in lotus leaf, significantly inhibits CYP2D6 (IC50: 3.76 μM, Ki: 2.34 μM).N-Nornuciferine strongly inhibits CYP2D6 activity but shows weak or no inhibition of the other four P450 isoenzymes (CYP2C19, CYP3A4, CYP2E1, CYP2C9). N-Nornuciferine competitively inhibits the CYP2D6-catalyzed dextromethorphan O-demethylation (Ki: 2.34 μM).
-
Azalanstat
Azalanstat (RS 21607) is an orally available selective mammalian lanosterol 14-alpha-demethylase inhibitor with hypocholesterolemic activity that inhibits cholesterol synthesis in HepG2 cells, human fibroblasts, hamster hepatocytes, and hamster livers through inhibition of the cytochrome P450 enzyme, lanosterol 14 alpha demethylase.
-
Alpha-Amyrin
Alpha-Amyrin is an inhibitor of trypsin and chymotrypsin with antineoplastic effects. alpha-Amyrin can be used in studies about acting as a hepatomodulatory.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


